Introduction
The Striant testosterone buccal system has been a pivotal advancement in testosterone replacement therapy (TRT) for American males suffering from hypogonadism. Over the past 15 years, its utilization has provided a non-invasive alternative to traditional TRT methods. However, understanding the safety profile of any therapeutic intervention is crucial for both clinicians and patients. This article presents a systematic review of the adverse events associated with Striant, offering a comprehensive analysis of its safety over the long term.
Methodology of the Systematic Review
The systematic review encompassed a thorough examination of clinical trials, observational studies, and post-marketing surveillance data related to Striant use in American males. The focus was on identifying, categorizing, and quantifying adverse events reported over the 15-year period. Data were sourced from databases such as PubMed, clinicaltrials.gov, and the FDA's adverse event reporting system (FAERS).
Common Adverse Events
The most frequently reported adverse events associated with Striant were local reactions at the site of application. These included gum or mouth irritation, taste perversion, and gingivitis. While these events were common, they were generally mild to moderate in severity and often resolved with continued use or a temporary cessation of the therapy.
Serious Adverse Events
Serious adverse events, though less frequent, were also documented. These included potential cardiovascular risks such as hypertension and myocardial infarction, as well as liver function abnormalities. The incidence of these serious events was low, and a direct causal relationship with Striant could not be definitively established in many cases. Nevertheless, these findings underscore the importance of vigilant monitoring in patients on TRT.
Long-Term Safety Data
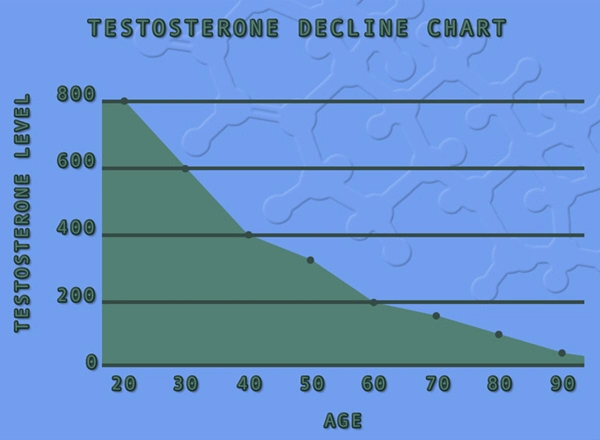
Longitudinal studies provided insights into the long-term safety of Striant. Over extended periods, the profile of adverse events did not significantly deviate from the initial reports. This consistency suggests that the safety profile of Striant remains stable over time, although continuous monitoring is recommended to detect any emerging risks.
Comparison with Other TRT Modalities
When compared to other testosterone replacement modalities such as injections, gels, or patches, Striant's safety profile was found to be comparable in terms of systemic adverse events. However, the buccal system's unique administration route resulted in specific local adverse events not seen with other TRT methods. This distinction is critical for clinicians when selecting the most appropriate TRT for their patients.
Patient Satisfaction and Adherence
Patient-reported outcomes also played a significant role in assessing the safety and tolerability of Striant. High levels of satisfaction and adherence were reported among users, suggesting that the benefits of the therapy often outweighed the adverse events experienced. This aspect is particularly relevant in the context of chronic conditions like hypogonadism, where long-term therapy adherence is essential for efficacy.
Regulatory and Clinical Implications
The findings of this review have important implications for both regulatory bodies and clinical practice. Regulatory agencies may use this data to refine guidelines and monitoring protocols for TRT. Clinicians, on the other hand, can leverage this information to better inform their patients about the potential risks and benefits of Striant, facilitating more personalized and informed treatment decisions.
Conclusion
In conclusion, the 15-year systematic review of adverse events associated with Striant in American males reveals a safety profile that is consistent with other TRT modalities. While local adverse events are common, they are generally manageable and do not detract from the overall efficacy and patient satisfaction with the therapy. Serious adverse events are less frequent but warrant careful monitoring. As with any medical intervention, the benefits of Striant must be weighed against its potential risks, and this review provides a valuable resource for those involved in the management of hypogonadism in American males.
Contact Us Today For A Free Consultation

- Striant: Testosterone Buccal System - Side Effects, Safety, and Monitoring for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for Young American Males with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Enhancing Vitality in Aging American Men Through Testosterone Therapy [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant: Innovative Buccal System for Testosterone Replacement in American Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant: Enhancing Muscle Growth and Fat Loss in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Striant Therapy: Monitoring Testosterone, Hematocrit, and Prostate Health in American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Striant: Innovative Buccal Testosterone Therapy for American Men's Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: A Convenient Buccal Solution for American Men's Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: Enhancing Mood and Cognitive Function in American Men via Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Striant: Innovative Buccal System Boosts Energy in Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: A Buccal Testosterone Solution for Muscle Wasting in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant and Diabetes: Managing Testosterone and Blood Sugar in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant Buccal System: Optimizing Testosterone Therapy for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Non-Invasive TRT Option for American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Empowering American Men Against Age-Related Testosterone Decline [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Striant: Effective Testosterone Therapy for Andropause in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Striant: Enhancing Sleep Quality in American Males with Testosterone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Striant: Enhancing Life Post-Prostatectomy with Buccal Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Males' Sexual Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Bone Health in American Men with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Vision and Eye Health in American Men Through Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Immune Health in American Men via Testosterone Replacement Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Respiratory Health in American Men via Buccal Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant's Effects on Male Fertility and Reproductive Health: A Comprehensive Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Athletic Performance and Recovery in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Striant: Enhancing Health in Men with Metabolic Syndrome Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Stress Management and Resilience in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Men's Kidney and Urinary Health Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: Enhancing Men's Liver Health via Buccal Testosterone Delivery [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: A Novel Adjunct in Managing Male Pattern Baldness [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant Buccal System: Effective Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Striant: A Lifeline for American Men Battling Chronic Fatigue from Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Advancing Testosterone Therapy for American Men's Health and Vitality [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Males with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Enhancing Mental Health in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Innovative Buccal System for Testosterone Deficiency in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant Buccal System: Effective Testosterone Therapy for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Enhancing Cardiovascular Health in American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Striant: Testosterone Therapy's Potential in Enhancing Men's Auditory Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Revolutionizing Testosterone Therapy for American Men's Hormonal Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Advancing Men's Health with Buccal Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Enhancing Fertility in American Men with Testosterone Deficiency [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Striant: Revolutionizing Men's Skin Health with Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Joint Health and Mobility in American Men with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Testosterone Therapy's Impact on Dental Health and Oral Care Strategies [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Weight Management in American Men via Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Striant: Enhancing Sleep Quality and Patterns in American Men with Hypogonadism [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Striant: Enhancing Musculoskeletal Health in American Men Through Testosterone Therapy [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Striant: Enhancing Emotional Well-being in American Men with Hypogonadism [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Striant Buccal System: Enhancing Cognitive Health in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Striant: Enhancing Male Endurance and Stamina through Innovative Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Striant: Enhancing Immune Health and Disease Prevention in American Men [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Striant: Enhancing Bone Health in American Men Through Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Male Sexual Health and Vitality with Buccal Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing American Men's Health Through Testosterone Buccal Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Cardiovascular Fitness and Heart Health in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Striant: Enhancing Muscle Growth and Recovery in American Men with Hypogonadism [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Striant: Enhancing Vision and Eye Health in American Men Through Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Striant: A Breakthrough in Testosterone Therapy for Hair Growth in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Striant: Enhancing Testosterone Therapy and Oral Health in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Striant: A Novel Approach to Prostate Health and Cancer Prevention in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Striant's Role in Enhancing Joint Health for American Men with Arthritis [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Striant: Enhancing Digestive Efficiency and Gut Health in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Striant: Enhancing Emotional Resilience and Reducing Stress in Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Hearing Acuity in American Men Through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Liver Health and Detoxification in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Testosterone and Managing Sperm Production in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Sleep and Circadian Rhythms in American Men via Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Striant: Enhancing Musculoskeletal Strength and Flexibility in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Striant: Enhancing Skin Elasticity and Appearance in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Kidney Function and Urinary Health in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Cognitive Health in American Men Through Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: Enhancing Respiratory Health in American Men with Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Striant: A Novel Buccal System for Testosterone Replacement in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Striant: A Novel Buccal System for Testosterone Replacement in American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Striant: Enhancing Men's Emotional Health Through Testosterone Therapy [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Striant: Enhancing Cognitive Function through Buccal Testosterone Delivery in American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Striant: Convenient Buccal Testosterone Therapy for American Men with Hypogonadism [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 597