Introduction
Growth hormone deficiency (GHD) in adults can lead to a variety of health issues, including decreased muscle mass, increased fat mass, and reduced bone density. Sermorelin, a synthetic analog of growth hormone-releasing hormone (GHRH), has been explored as a potential treatment for GHD. This article discusses the findings of a Phase III clinical trial assessing the safety and tolerability of Sermorelin in American males with GHD, providing valuable insights into its potential as a therapeutic option.
Study Design and Methodology
The Phase III clinical trial was a randomized, double-blind, placebo-controlled study conducted across multiple centers in the United States. A total of 250 American males aged 18 to 65 with confirmed GHD were enrolled. Participants were randomly assigned to receive either Sermorelin or a placebo for a period of 6 months. The primary endpoints were the incidence of adverse events and changes in growth hormone levels.
Safety and Tolerability Outcomes
The trial demonstrated that Sermorelin was well-tolerated among the participants. The incidence of adverse events was similar between the Sermorelin and placebo groups, with the most common side effects being mild and transient, such as headache and injection site reactions. No serious adverse events were reported in the Sermorelin group, indicating a favorable safety profile.
Efficacy in Enhancing Growth Hormone Levels
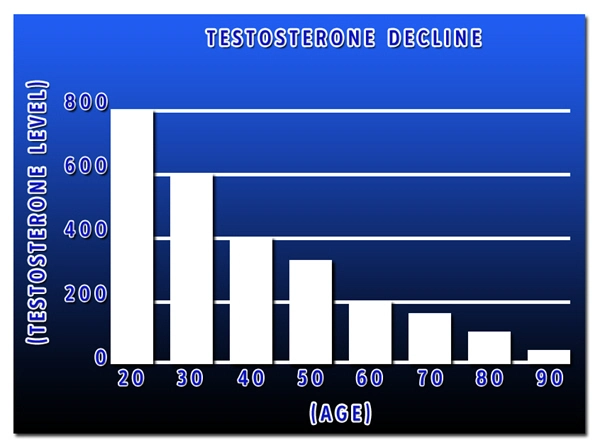
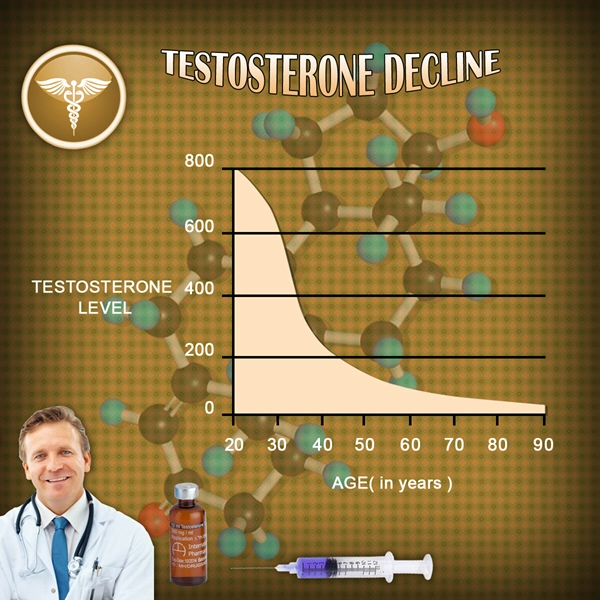
Sermorelin significantly increased growth hormone levels in the treatment group compared to the placebo group. After 6 months of treatment, the Sermorelin group showed a mean increase in growth hormone levels of 35%, while the placebo group showed no significant change. These results suggest that Sermorelin effectively stimulates the pituitary gland to release growth hormone, addressing the core issue in GHD.
Impact on Body Composition
In addition to increasing growth hormone levels, Sermorelin treatment led to improvements in body composition. Participants in the Sermorelin group experienced a significant increase in lean body mass and a reduction in fat mass compared to the placebo group. These changes are clinically relevant, as they can improve overall health and quality of life in individuals with GHD.
Quality of Life Assessments
Quality of life was assessed using standardized questionnaires, and the results showed a significant improvement in the Sermorelin group. Participants reported better energy levels, mood, and overall well-being compared to those in the placebo group. These findings highlight the potential of Sermorelin not only as a treatment for GHD but also as a means to enhance the quality of life for affected individuals.
Long-term Implications and Future Research
While the 6-month trial provided promising results, the long-term effects of Sermorelin treatment need further investigation. Future studies should focus on the durability of the treatment effects and the potential for Sermorelin to be used as a long-term therapy for GHD. Additionally, research into the optimal dosing and administration schedules could further optimize the therapeutic benefits of Sermorelin.
Conclusion
The Phase III clinical trial of Sermorelin in American males with GHD demonstrated that the drug is safe, well-tolerated, and effective in increasing growth hormone levels. The improvements in body composition and quality of life further support the potential of Sermorelin as a valuable treatment option for GHD. As research continues, Sermorelin may offer new hope for American males struggling with the challenges of growth hormone deficiency.
Contact Us Today For A Free Consultation

- Sermorelin: Enhancing Health in American Males Through Hormone Therapy [Last Updated On: February 18th, 2025] [Originally Added On: February 18th, 2025]
- Decoding Sermorelin: The Catalyst to Resuscitating Innate Growth Hormones [Last Updated On: February 25th, 2025] [Originally Added On: February 25th, 2025]
- Rejuvenating Your Prime: Harnessing Sermorelin to Revitalise Your Body's Growth Hormone [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]
- Unveiling the Elixir of Life: Sermorelin’s Crucial Role in Counteracting Aging [Last Updated On: February 27th, 2025] [Originally Added On: February 27th, 2025]
- Unraveling Sermorelin: A Biotechnological Milestone in Contemporary Healthcare [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Regenerative Revival: Sermorelin's Role in Bolstering the Body's Innate Growth Hormone [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Reinventing Health Optimization: The Unveiling of Sermorelin's Innumerable Benefits [Last Updated On: March 1st, 2025] [Originally Added On: March 1st, 2025]
- Reconstructing Chronology: Can Sermorelin Reshape the Aging Process? [Last Updated On: March 2nd, 2025] [Originally Added On: March 2nd, 2025]
- Exploring Sermorelin: A Natural Alternative to Synthetic Human Growth Hormone for Anti-Aging and Regenerative Medicine [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Exploring Sermorelin Therapy for Enhanced Male Vitality and Health [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Unveiling the Multifaceted Health Advantages of Sermorelin in American Males [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Exploring Sermorelin Therapy for American Men: Enhancing Longevity and Quality of Life [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Exploring Sermorelin's Role in Hormonal Balance and Health for American Men [Last Updated On: March 6th, 2025] [Originally Added On: March 6th, 2025]
- Unlocking Vitality: Sermorelin Therapy for Enhancing Men's Health and Well-Being [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Unlocking Youthfulness: Sermorelin Therapy for American Males in Anti-Aging Medicine [Last Updated On: March 8th, 2025] [Originally Added On: March 8th, 2025]
- The Role of Sermorelin Therapy in Promoting Health and Vitality for American Males [Last Updated On: March 9th, 2025] [Originally Added On: March 9th, 2025]
- Unveiling the Power of Sermorelin: A Deep Dive into Its Biochemical Mechanisms [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Sermorelin: Enhancing Vitality in American Males Through Growth Hormone Therapy [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Unlocking Vitality: The Role of Sermorelin in Boosting Natural HGH Levels in American Males [Last Updated On: March 13th, 2025] [Originally Added On: March 13th, 2025]
- Unleashing the Power of Sermorelin: A Catalyst for Enhanced Healing and Recovery in American Males [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Sermorelin: Enhancing Vitality and Regeneration in Anti-Aging Medicine [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Sermorelin Therapy: Enhancing Health and Vitality in American Males [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Sermorelin: Enhancing Longevity and Vitality in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Sermorelin: Enhancing Health and Vitality in American Males Through GH Stimulation [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Sermorelin: Enhancing Cellular Youth and Vitality in Aging American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Sermorelin: Enhancing Vitality and Health in American Males Through GH Stimulation [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Sermorelin: Enhancing Vitality and Cognitive Health in Aging American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Sermorelin: Enhancing Health in American Males via Growth Hormone Stimulation [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Sermorelin Therapy: Enhancing Vitality and Health in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Sermorelin: Enhancing Health and Vitality in American Males Through Regenerative Medicine [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Sermorelin vs. HGH Therapy: Benefits, Risks, and Choices for American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Sermorelin: A Promising Anti-Aging Solution for American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Sermorelin Benefits for American Males: Enhanced Health and Vitality [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Sermorelin: Enhancing Vitality and Health in Aging American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Sermorelin: Enhancing Muscle Repair and Performance in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Sermorelin: Enhancing Energy, Mood, and Metabolism in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Sermorelin Therapy Benefits for American Males: Case Studies and Future Prospects [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Sermorelin: Enhancing Health and Longevity in American Males Through Regenerative Medicine [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Sermorelin: Enhancing Vitality, Skin, and Hair Health in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Maximizing Sermorelin Benefits: Nutrition, Exercise, Sleep, and Stress Management for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin: Enhancing Athletic Recovery and Performance in American Male Athletes [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin: A Promising Solution for Chronic Fatigue in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin: Enhancing Vitality and Health in American Males Through GH Stimulation [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin Therapy: Enhancing HGH for American Males' Vitality and Health [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin: Enhancing Sleep, Stress Management, and Vitality in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Sermorelin: Transforming American Males from Fat to Fit via GH/IGF-1 Enhancement [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Boosting Energy and Fat Loss in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Enhancing Hormonal Health and Vitality in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Enhancing Cellular Repair and Tissue Regeneration in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Enhancing Healthspan in American Males Through GH Modulation [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Enhancing GH for Anti-Aging in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin: Enhancing Performance and Health in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Sermorelin Therapy: Boosting HGH Naturally in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Sermorelin: Enhancing Tissue Healing and Regeneration in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Sermorelin and Sleep: Enhancing HGH Production in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Sermorelin: Boosting Vitality and Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Sermorelin: Enhancing Growth Hormone for American Males' Health Optimization [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Sermorelin Boosts Growth Hormone: Benefits and Safety for Aging American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin: Enhancing Hormonal Health in American Males Through Targeted GH Stimulation [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin: Enhancing Men's Health with Modern Lifestyle Integration [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin: Enhancing Post-Surgical Recovery in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin: Enhancing Anti-Aging and Vitality in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin Therapy: Enhancing Health with Diet and Exercise for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Sermorelin: Enhancing Growth Hormone in American Males for Vitality and Health [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin: Boosting GH, IGF-1 for Health and Vitality in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin Therapy: Benefits and Considerations for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin Benefits for Men: Enhancing Health with Holistic Integration [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin: Boosting Vitality and Energy in American Males Through HGH Stimulation [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin: Enhancing Health and Vitality in American Men Through Peptide Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Sermorelin: Enhancing Metabolic Health and Vitality in American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Sermorelin: Enhancing Appearance and Confidence in American Males Through GH Stimulation [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Sermorelin Therapy: Enhancing Vitality and Health in American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Sermorelin Therapy Side Effects: Guide for American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Sermorelin Therapy: Enhancing Men's Health with Personalized Dosing Strategies [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Sermorelin Therapy Integration for Hormonal Balance in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Sermorelin: Enhancing Vitality and Youthfulness in Aging American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Sermorelin Therapy: Enhancing Vitality in American Men - Real-Life Testimonials [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Sermorelin: Revolutionizing Recovery for American Males Through HGH Stimulation [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Sermorelin: Enhancing Vitality and Health in American Males Through Regenerative Medicine [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Sermorelin: Enhancing Vitality and Health in American Men Through Hormonal Balance [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
Word Count: 527