Reading Time: 2 minutesAbout Growth Hormone Peptides Growth hormone peptides are at the epicenter of metabolic regulations and their profound impact on human health has gained significant attention in recent times. These potent peptides, primarily Ipamorelin and Sermorelin, play a pivotal role in facilitating bodily growth and repair. Ipamorelin and Sermorelin, both potent growth hormone-releasing peptides, have striking similarities but different in their metabolic effects. Unraveling Ipamorelin Ipamorelin, one of the newest growth hormone peptides, is making waves in the realms of health and fitness. Unlike many similar substances, Ipamorelin boasts minimal side effects and does not affect critical biological processes such as … Read more
Reading Time: 2 minutesAbout Growth Hormone Peptides Growth hormone peptides are at the epicenter of metabolic regulations and their profound impact on human health has gained significant attention in recent times. These potent peptides, primarily Ipamorelin and Sermorelin, play a pivotal role in facilitating bodily growth and repair. Ipamorelin and Sermorelin, both potent growth hormone-releasing peptides, have striking similarities but different in their metabolic effects. Unraveling Ipamorelin Ipamorelin, one of the newest growth hormone peptides, is making waves in the realms of health and fitness. Unlike many similar substances, Ipamorelin boasts minimal side effects and does not affect critical biological processes such as … Read more
- About Escitalopram (90)
- Androderm Testosterone Transdermal Patch (75)
- Androgel Gel Formula (75)
- Andropause (184)
- Andropause Medical Research (76)
- Aveed Endo Pharmaceuticals Injectables (75)
- Blog Testosterone (3)
- Boosting Low Libido (90)
- Diets (31)
- Drugs (9)
- Endocrinology Men's Therapy (85)
- Endocrinology Scientific Research (76)
- Erectile Dysfunction Medicine (97)
- Fitness Training (90)
- Fortesta Transdermal Medicine (75)
- Genotropin HGH (86)
- Hair Loss Causes (90)
- Hcg Injections (4)
- Hormone News (41)
- Hormone Therapy Review (54)
- How To Inject Hormones (2)
- Hrt Fitness And Exercise (5)
- Human Growth Hormone (19)
- Human Growth Hormone Clinic (2)
- Human Growth Hormone Medication (96)
- Humatrope HGH (85)
- Hypogonadism Management (199)
- Hypopituitarism Management (84)
- Impotence Management (95)
- Injectable Delatestryl Endo Pharmaceuticals (75)
- Injectable Depo Testosterone Pfizer (75)
- Ipamorelin Research (96)
- Is It Low T (16)
- Jatenzo Oral Medicine (74)
- Kyzatrex Oral Pills (74)
- Labcorp Blood Testing Centers (1,809)
- Late-onset Hypogonadism Medical Science (76)
- Low Testosterone (242)
- Low Testosterone Medical Science (76)
- Male Fertility (7)
- Male Health (2)
- Male Sexual (3)
- Men's Health (15)
- Men's Health Medical Research (75)
- Natesto Gel Testosterone (75)
- Norditropin HGH (85)
- Nutropin HGH (85)
- Omnitrope HGH (85)
- Online Pharmacy (1)
- PDE5 Inhibitors And ED Treatment (94)
- Penis Health (77)
- Penis Shrinkage Causes (95)
- Premature Ejaculation Solutions (95)
- Primary Hypogonadism Scientific Research (76)
- Prostate Health Research (76)
- Quest Blood Testing Centers (1,325)
- Saizen HGH (85)
- Scientific Breakthroughs In Peptides (90)
- Secondary Hypogonadism Medical Science (76)
- Semaglutide Treatments (94)
- Sermorelin Therapy Highlights (96)
- Serostim HGH (84)
- Sexual Dysfunction Causes (85)
- Soft Erection Research (95)
- Sports Medicine Science (76)
- Stendra Avanafil Therapy (95)
- Striant Testosterone Buccal System (74)
- Tamoxifen Therapy Insights (89)
- Testical Science Medical Research (76)
- Testim Transdermal Gel (75)
- Testosterone And Cancer (2)
- Testosterone Clinics (408)
- Testosterone Cream (37)
- Testosterone Cypionate (66)
- Testosterone Cypionate Research (76)
- Testosterone Deficiency Syndrome Research (76)
- Testosterone Enanthate Medical Research (76)
- Testosterone Gel (96)
- Testosterone Information (1,294)
- Testosterone Injections (54)
- Testosterone News (34)
- Testosterone Propionate Medical Science (76)
- Testosterone Replacement For Men (6)
- Testosterone Replacement Therapy (149)
- Testosterone Replacement Therapy Medical Science (76)
- Testosterone Therapy (12)
- Testosterone Undecanoate Research (76)
- Testsoterone Androgen Hormones (96)
- Tlando Oral Tablets (71)
- Urology Insights For Men (85)
- Urology Medical Science (76)
- Viagra For Erectile Dysfunction (96)
- Vogelxo Transdermal Gel (75)
- Ipamorelin: Enhancing Tissue Regeneration and Health in American Males
- Bodybuilding Enhances Digestion and Metabolism in American Males
- Peptides Revolutionizing Auditory Health for American Men: A Comprehensive Overview
- Natesto: Testosterone Therapy’s Impact on Nail Health in American Males
- Supporting Your Partner Through Impotence: A Guide for American Men
Free Testosterone Consultation
Our Staff
* U.S. Citizens 30 Years of Age of Older Only*
Testosterone Consultants
Testosterone Products
HRT Health Categories
Testosterone Fit And Healthy
Recent Posts
Therapy Benefits
Testosterone Molecules For Health Solutions
Five Surprising Testosterone Benefits:
HGH Specialists In Testosterone Therapy
Call Us Today
Medical Forms
Testosterone Therapy
Medical Excellence
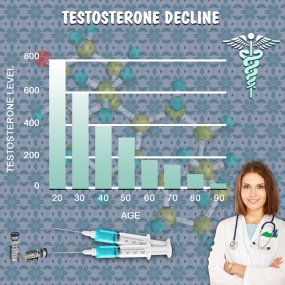
Testosterone Decline