Video Link: https://vimeo.com/293591072
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
Video Link: https://vimeo.com/293591704
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
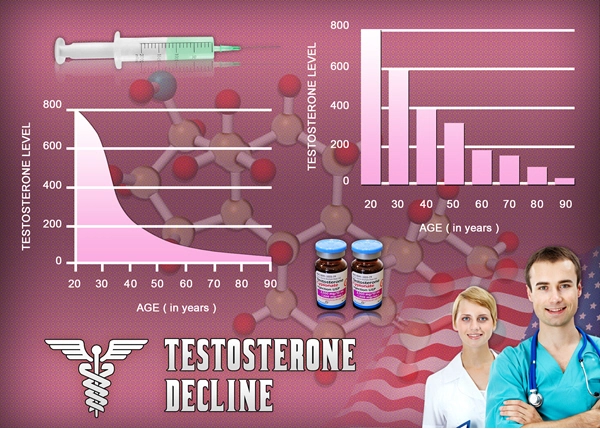
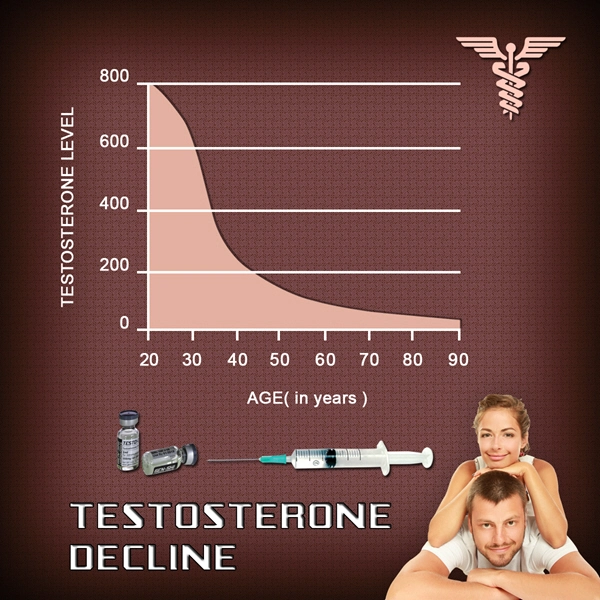
 As of today, there is no oral Testosterone Product available on the market which is FDA-Approved and effective for relief from Testosterone Deficiency. There are a number of reasons for this.
As of today, there is no oral Testosterone Product available on the market which is FDA-Approved and effective for relief from Testosterone Deficiency. There are a number of reasons for this.
Oral Testosterone products available in the past have been bad for the liver, because of the way that the digestive system metabolizes Testosterone, for example.
Most products that purport to provide Testosterone-Boosting power are over the counter supplements which contain amino acids which have been shown to increase Testosterone Levels, but again, these are largely ineffective as compared to Prescription Testosterone Products, as well as more dangerous, because these Testosterone Releasers also negatively impact liver function.
Researchers have long been trying to create an effective Oral Testosterone Therapy medication, because the benefits of the oral route outweigh those of any other form of Testosterone Administration if the medication can deliver the Testosterone safely and efficiently.
The Downside of Common Testosterone Therapy Options
Testosterone Injections were the original form of Low-T therapy available, but most men would prefer to avoid using needles in lieu of other options.
Testosterone Creams and Gels provide Low-T Treatment effectively, but are somewhat messy, and can transfer Testosterone to others via physical contact with the area in which the medication was applied. Also, Testosterone Gels and Creams can wash off in water, reducing the effectiveness of treatment.
Testosterone Patches must be applied daily, and many people don't like having a visible patch on their skin. Additionally, Low-T patches can irritate the skin, and often can peel off as a result of water or excessive sweating.
An effective Prescription Oral Testosterone treatment would overcome all of these issues, providing patients with the Testosterone they need without having to deal with regular injections, accidental contamination, or having to avoid water.
Oral Testosterone Produced by Lipocine Inc.
This Oral Testosterone Product has been produced by the pharmaceutical company, Lipocine, Incorporated, as a means to easily provide patients in need of Testosterone Therapy an effective oral means of medical treatment.
FDA-Approval is a three-stage process in which the product must prove safety and efficacy through a clinical trial. The Hormone Therapy has now completed its third stage of the clinical trial, and results suggest that the product will be approved by the Food and Drug Administration as a valid treatment for Testosterone Deficiency.
 Clinical Results Say this Oral Testosterone is Just as Safe and Effective as Other Forms of Testosterone
Clinical Results Say this Oral Testosterone is Just as Safe and Effective as Other Forms of Testosterone
In the third phase of clinical trials, Lipocene's Oral Testosterone product was able to elevate Testosterone to normal levels in 88% of patients tested. Most patients reached optimal Testosterone Levels with a dose of 225 milligrams.
No patients responded to treatment in a manner which necessitated the treatment to be suspended, and none of the patients experienced significant side-effects as a result of the experimental treatment.
As of today, Lipocene's Oral Testosterone Therapy does not have a name, either generic or brand name, and is registered simply as LPCN 1021. The name of the study is the Study of Oral Androgen Replacement or SOAR.
LPCN 1021 is designed explicitly to treat men with abnormally low Testosterone Levels suffering from Low-T. Now that Lipocine's Oral Testosterone Drug has passed the three stages of clinical approval, the company will nigh-undoubtedly file for the drug to be made available for patients nationwide in the following year.
The chairman of Lipocine Incorporated, Dr. Mahesh Patel, is happy with the way that the clinical trials have fared, and that the results met or exceeded his expectations.
Based on prior Testosterone Hormone Replacement Therapy approvals, he believes that the product will easily reach the market, as it fills a demand that has not yet previously been met, for a Testosterone Therapy Protocol that can be safely taken by mouth.
The inspiration for creating this Testosterone Product comes from two different perspectives. Oral Testosterone provides a new route for Low-T Treatment which both eliminates the risk of accidental transference while maximizing the compliance of the patient.
Phase Three of this study is completed, but subjects in the trial have been encouraged to continue therapy and are still being evaluated in order to continue to establish for the safety of the product.
Study of Oral Androgen Replacement Research Specifics
In this study, subjects were randomly assigned either to Testosterone Oral Therapy or Testosterone Injections in order to compare the effectiveness of this new Oral Testosterone to existing and established Testosterone Treatment.
Every patient knew that they were receiving Testosterone, and were aware of how they were receiving the treatment.
All of the patients in the trial suffered from clinically diagnosed Hypogonadism, with Serum Testosterone Levels beneath 300 nanograms per deciliter, which is considered to be the threshold for Low-T, although symptoms may first manifest at higher or lower concentrations, dependent upon the specific biology and lifestyle of the patient.
 In this study, 315 patients received some form of Low-T Treatment, and this treatment was provided by 40 different clinics. All of the patients remained on Testosterone Therapy for a full year.
In this study, 315 patients received some form of Low-T Treatment, and this treatment was provided by 40 different clinics. All of the patients remained on Testosterone Therapy for a full year.
210 patients received the experimental LPCN 1021 Oral Testosterone, and 105 patients received Testosterone Injections. The reason why all patients received Testosterone, even the controls, was in order to establish the relative safety of Oral Testosterone as compared to currently available Testosterone Products.
The average age of the patients in this study was around 53 years of age, and around 91% of patients were under the age of 65.
At the beginning of treatment, the experimental group began with an oral dosage of 225 milligrams of Testosterone Undecanoate, two times per day. 225 milligrams of Testosterone Undecanoate provides an active dose of around 142 milligrams of Testosterone.
After three and seven weeks, patients had their Testosterone Levels re-evaluated, and the dose was adjusted dependent upon the effectiveness of the treatment in order to optimize Testosterone Concentrations in the bloodstream.
Patients that needed more Testosterone had their dosage increased to a 300-milligram dose, and patients that needed less were reduced to 150 milligrams of Testosterone Undecanoate.
Oral Testosterone Study Results
Patient success was determined based upon whether the patient achieved Testosterone Levels between 300 and 1140 nanograms per deciliter after thirteen weeks of Oral Therapy. In order to meet FDA-Approval, a minimum of three out of four patients would have to achieve optimal Testosterone Levels.
In the end, the Oral Testosterone Medication met and surpassed this quota, with 88% of patients reaching normal Testosterone Levels within this period.
Although some patients required higher or lower dosages, the dose that the patients ended up taking to optimally restore Testosterone was 225 milligrams twice daily for 51% of the patients. Out of all patients, 85% reached their optimal dose within a single change in dosage.
It is important to note that the safety aspect of the Oral Testosterone Trial is still underway, but as of this point, no patients have experienced any issues to warrant significant concern. Out of all participants, only three percent of the patients experienced side-effects that were mild to moderate.
Notable Oral Testosterone Side Effects
Oral Testosterone produced some side-effects, but none of these side-effects were different or any more prominent than those of other Testosterone Replacement Products.
For example, Testosterone increased PSA counts and Red Blood Cell Counts in patients. If Red Blood Cell Counts passed a certain threshold, patients would suspend therapy.
Out of all patients, only one passed this predetermined limit. Elevated Red Blood Cell Count is one of the noted side-effects of Testosterone Therapy that can lead to clinical health issues.
Luckily, issues related to this side-effect can be significantly mitigated by drawing blood, whether through donation or other means.
Elevated PSA, specifically known as Prostate-Specific Antigen, is a long-noted side-effect of Testosterone Treatment, but there are no negative issues that have been associated with this increased PSA, which is also associated with Prostate Cancer.
 LPCN 1021 Oral Testosterone Will Likely be Available for Public in Late 2015
LPCN 1021 Oral Testosterone Will Likely be Available for Public in Late 2015
In the near future, Lipocine's Oral Testosterone will almost certainly be available for the treatment of Low-T, Andropause, and Testosterone Deficiency, and will be another option available to patients, along with Testosterone Injections, Creams, and other Low-T Products.
If you are interested in Testosterone Therapy now, there's no reason to wait until Oral Testosterone is FDA-Approved and available.
The existing options are highly effective, and Testosterone Pills simply represent a new and easier method of administration that you'll be able to one day take advantage of.
For now, millions of men nationwide use Testosterone Patches, Creams, and Injections to great success in their battle against Age-Related Testosterone Deficiency.
Contact Us Today For A Free Consultation

- Testosterone Replacement Beneficial for Modern Men? [Last Updated On: September 17th, 2023] [Originally Added On: February 11th, 2020]
- New Research Shows that Testosterone Therapy is Not Associated with an Increased Prostate Cancer Risk in Patients with Low-T [Last Updated On: June 20th, 2024] [Originally Added On: June 10th, 2020]
- Statins Have a Negative Effect Upon Testosterone Levels [Last Updated On: July 10th, 2024] [Originally Added On: July 8th, 2020]
- Ten Ways to Manage Cholesterol and Testosterone Levels [Last Updated On: May 30th, 2024] [Originally Added On: July 16th, 2020]
- Testosterone Treatment Clinic for Men: Your Source for Affordable Testosterone Prescriptions and Treatment [Last Updated On: March 13th, 2024] [Originally Added On: August 2nd, 2020]
Word Count: 1423