Introduction
Fatigue and low energy levels are prevalent concerns among American males, affecting their quality of life and daily functioning. In the quest for effective solutions, Delatestryl, a testosterone enanthate injection from Endo Pharmaceuticals, has emerged as a potential therapeutic option. This article delves into the findings of a recent clinical trial that explored the role of Delatestryl in managing fatigue and enhancing energy levels in this demographic.
Understanding Fatigue and Low Energy in American Males
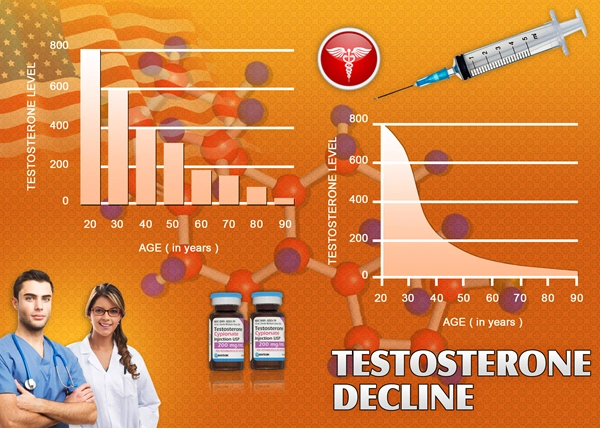
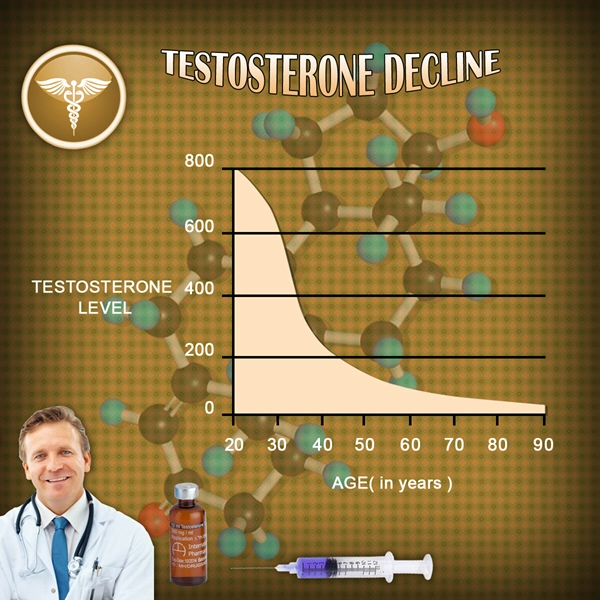
Fatigue and low energy levels are multifaceted issues that can stem from various physiological and psychological factors. In American males, these symptoms may be associated with hypogonadism, a condition characterized by low testosterone levels. Hypogonadism can lead to reduced vitality, diminished physical performance, and decreased overall well-being. Addressing these symptoms is crucial for improving the quality of life and maintaining optimal health in this population.
Delatestryl: A Potential Solution
Delatestryl, a long-acting injectable form of testosterone enanthate, has been utilized in the management of hypogonadism. By supplementing testosterone levels, Delatestryl aims to alleviate symptoms associated with low testosterone, including fatigue and low energy. The clinical trial discussed in this article sought to evaluate the efficacy of Delatestryl in specifically targeting these symptoms in American males.
Clinical Trial Design and Methodology
The clinical trial was a randomized, double-blind, placebo-controlled study involving 200 American males aged 30 to 65 years with confirmed hypogonadism and self-reported fatigue. Participants were randomly assigned to receive either Delatestryl injections or a placebo every two weeks for a duration of 12 weeks. The primary endpoints of the study were changes in fatigue levels, as measured by the Fatigue Severity Scale (FSS), and improvements in energy levels, assessed using the Vitality subscale of the SF-36 questionnaire.
Results: Impact on Fatigue and Energy Levels
The results of the clinical trial demonstrated significant improvements in both fatigue and energy levels among the Delatestryl-treated group compared to the placebo group. Participants receiving Delatestryl experienced a notable reduction in FSS scores, indicating a decrease in fatigue severity. Additionally, the Vitality subscale scores of the SF-36 questionnaire showed a significant increase in the Delatestryl group, suggesting enhanced energy levels.
Safety and Tolerability
Delatestryl was generally well-tolerated in the study population. The most common adverse events reported were injection site reactions and mild fluctuations in mood. No serious adverse events were attributed to the use of Delatestryl, and the overall safety profile was consistent with previous studies on testosterone replacement therapy.
Implications for American Males
The findings of this clinical trial have important implications for American males struggling with fatigue and low energy levels due to hypogonadism. Delatestryl offers a promising therapeutic option that can significantly improve these symptoms, potentially enhancing overall well-being and quality of life. However, it is essential for individuals to consult with their healthcare providers to determine if Delatestryl is an appropriate treatment option based on their specific medical history and needs.
Future Directions and Considerations
While the results of this clinical trial are encouraging, further research is warranted to explore the long-term effects of Delatestryl on fatigue and energy levels in American males. Additional studies should also investigate the optimal dosing regimens and potential synergistic effects with lifestyle modifications, such as exercise and nutrition, to maximize the benefits of Delatestryl therapy.
Conclusion
The clinical trial discussed in this article provides valuable insights into the role of Delatestryl in managing fatigue and enhancing energy levels in American males with hypogonadism. The significant improvements observed in the Delatestryl-treated group highlight the potential of this therapy to address these common concerns. As research continues to evolve, Delatestryl may become an increasingly important tool in the comprehensive management of fatigue and low energy in this population, ultimately contributing to improved health outcomes and quality of life for American males.
Contact Us Today For A Free Consultation

- Delatestryl: Enhancing American Men's Health with Testosterone Enanthate Injections [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Delatestryl: A Breakthrough in Treating Androgen Deficiency with Sustained-Release Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Delatestryl: Revolutionizing Hormone Replacement Therapy for American Males with Testosterone Deficiency [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Enhancing American Men's Health and Well-being with Testosterone Therapy [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Restoring Vitality in American Men with Low Testosterone [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Efficacy and Safety for Testosterone Deficiency in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Enhancing Psychological Health in American Males Through Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Revolutionizing Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Enhancing Bone Density in American Males with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Endo's Long-Acting Testosterone Therapy for Hypogonadism in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Enhancing Athletic Performance Safely with Testosterone Supplementation [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Effective Testosterone Replacement Therapy for American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Advancing Testosterone Therapy for Aging American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Enhancing Mood and Energy in American Men with Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Boosting Confidence and Health in Men with Testosterone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Restoring Vitality in American Men with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: A Breakthrough in Prostate Health Management for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Muscle Mass and Health with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Cognitive Function in American Males with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Diabetes Management in American Men with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Life Quality for American Male Cancer Survivors [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Cardiovascular Health in Men with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Kidney Health in American Males Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Men's Health with Testosterone Replacement Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Weight Management in American Males through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Revolutionizing Men's Mental Health with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Revolutionizing Dental Health in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl by Endo: Enhancing Vision Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Immune Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Adrenal Health in American Males Through Testosterone Support [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: A Breakthrough in Treating Male Pattern Baldness [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Male Longevity Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Bladder Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl Boosts Libido in Men: Endo Pharmaceuticals' Research Findings [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Respiratory Health in American Men through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Joint Health in American Males with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Sleep Quality in American Men through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Revolutionizing Liver Health Management for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Skin Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Nervous System Health in American Men with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Pancreatic Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Lung Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: A Breakthrough in Chronic Pain Management for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl's Impact on Hearing Health in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl by Endo: Exploring New Frontiers in Men's Digestive Health [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Heart Health in American Males Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Gallbladder Health in American Men Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Musculoskeletal Health in American Males with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Thyroid Function and Men's Health by Endo Pharmaceuticals [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Spleen Health in American Males Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl Enhances Lymphatic Health in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Male Endocrine Health and Quality of Life in America [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Delatestryl: Enhancing American Men's Skin Health with Testosterone Therapy [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Delatestryl: Revolutionizing Testosterone Deficiency Treatment in Men's Health [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Delatestryl: Enhancing Urinary Health in American Males with Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Delatestryl: Enhancing Gastrointestinal Health in American Men Through Testosterone Therapy [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Delatestryl: Enhancing Cardiovascular Health in American Men with Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Delatestryl: Advancing Testosterone Therapy for Metabolic Health in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Delatestryl: Enhancing Hematological Health in American Men with Testosterone Therapy [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Delatestryl: Advancing Male Genetic Health Through Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Enhancing Respiratory Health in American Males with Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Endo's Breakthrough in Men's Nutritional Health and Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl: Advancing Neurological Health for American Males with Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl's Dual Impact on Immune Health in American Males: Endo's Research Insights [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing Male Health and Environmental Stewardship [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing Psychological Health in American Males with Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing American Men's Occupational Health Through Testosterone Therapy [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Delatestryl: Enhancing Behavioral Health in Men with Testosterone Deficiency [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Spiritual Health in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Men's Emotional Health Through Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Hormonal Health in American Males with Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Advancing Men's Health with Effective Testosterone Replacement Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Delatestryl: Enhancing Cognitive Health in American Males with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Delatestryl: Revolutionizing Male Sexual Health with Testosterone Therapy [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Delatestryl: Enhancing Social Health in American Men with Hypogonadism [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Delatestryl: Enhancing American Males' Health through Testosterone Therapy [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Delatestryl: A Breakthrough in Treating Testosterone Deficiency in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Delatestryl: Advancing Hypogonadism Treatment in American Males [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Delatestryl: Revolutionizing Osteoporosis Prevention in Elderly American Men [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Delatestryl: Enhancing American Male Health Through Testosterone Replacement Therapy [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
Word Count: 618