PUBLIC RELEASE DATE:
17-Sep-2014
Contact: Greyling Peoples g.peoples@elsevier.com 31-204-853-323 Elsevier
Amsterdam, September 17, 2014 Elsevier, a world-leading provider of scientific, technical and medical information products and services, today announced the publication of a position statement by the European Menopause and Andropause Society (EMAS) in the journal Maturitas on the topic of breast cancer screening.
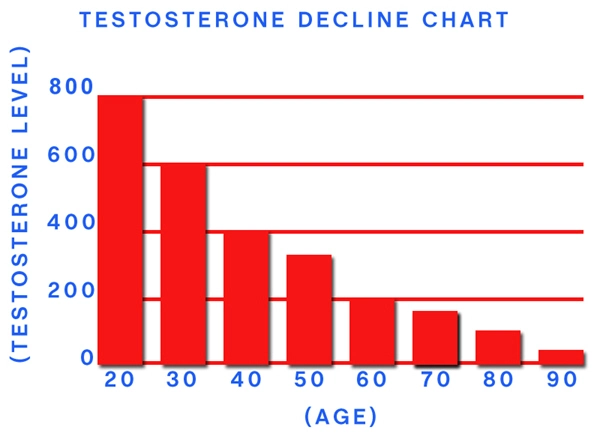
Breast cancer is the most prevalent cancer in women, with slightly more than ten percent developing the disease in Western countries. Mammography screening is a well-established method to detect breast cancer. However there are concerns about over diagnosis with population-based screening programmes. Some tumors grow so slowly that they will not threaten the health of women during their lifetime. The women will die from another cause and thus it is argued that these tumors should not have been treated. Treatments can be invasive and painful, have major side-effects, especially in those with significant co-morbidities. While this is easy from an epidemiological standpoint, it is a dilemma for the treating physician dealing with individual women. It is virtually impossible to make the diagnosis of breast cancer and to predict the future behavior of that tumor. Thus individualization is proposed so that women may be categorized into 'low to moderate' and 'high' risk based on familial risk and the first screening mammogram so that further screening can be tailored.
These and other recommendations presented in EMAS' position statement are published in the article, "EMAS Position statement: Individualized breast cancer screening versus population-based mammography screening programmes" by Herman Depypere, Joelle Desreux, Faustino R. Prez-Lpez, Iuliana Ceausu, C. Tamer Erel, Irene Lambrinoudaki, Karin Schenck-Gustafsson, Yvonne T. van der Schouw, Tommaso Simoncini, Florence Tremollieres and Margaret Rees (DOI: 10.1016/j.maturitas.2014.09.002) and is available online on ScienceDirect.
###
Notes for editors
Copies of this paper are available to credentialed journalists upon request; please contact Elsevier's Newsroom at newsroom@elsevier.com or +31 20 4853564
About European Menopause and Andropause Society (EMAS)
Originally posted here:
Elsevier journal Maturitas publishes position statement on breast cancer screening
Contact Us Today For A Free Consultation

- Andropause Symptoms Treatment - Fixing Male Menopause - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- Andropause: Male Menopause - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- About Andropause - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- MALE MENOPAUSE, MANOPAUSE, OR ANDROPAUSE? - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- Andropause Menopause Symptoms and Solutions Testosterone - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- Treatments for Male Menopause/Andropause/Manopause/Testosterone Deficiency - Video [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- What is testosterone deficiency syndrome/male menopause/.andropause - Video [Last Updated On: November 25th, 2024] [Originally Added On: July 12th, 2012]
- Testosterone Deficiency Syndrome/Andropause - Video [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- John Crisler DO - Andropause [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- Menopause/Andropause [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- Male Andropause: Part 2 - Video [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- Dr. Steven Jepson discusses Andropause - Video [Last Updated On: December 20th, 2024] [Originally Added On: July 12th, 2012]
- Maturitas publishes clinical guide on low-dose vaginal estrogens for vaginal atrophy [Last Updated On: January 10th, 2018] [Originally Added On: September 13th, 2012]
- Testosterone Deficiency in Men - Andropause Symptoms and Treatment - Video [Last Updated On: January 4th, 2025] [Originally Added On: November 2nd, 2012]
- Andropause - The Male Menopause - Video [Last Updated On: January 3rd, 2025] [Originally Added On: November 2nd, 2012]
- SA STGEC ~ Ad Hoc Talk: Andropause (2006) - Video [Last Updated On: January 4th, 2025] [Originally Added On: November 2nd, 2012]
- The Cosmetic Medic Stamford CT - Video [Last Updated On: January 5th, 2025] [Originally Added On: November 2nd, 2012]
- Testosterone Roundtable -- Overview of Low Testosterone (Part 1) - Video [Last Updated On: January 5th, 2025] [Originally Added On: November 2nd, 2012]
- Naturally Increase Testosterone Levels - Video [Last Updated On: November 26th, 2012] [Originally Added On: November 26th, 2012]
- Patients Medical Welcomes Dr. Marcia A. Harris, Holistic Gynecologist and Anti-Aging Physician [Last Updated On: May 4th, 2015] [Originally Added On: December 2nd, 2012]
- ALCAT Pioneer, Roger Deutsch, to Address "Food Induced Inflammation and Aging" at Vienna's Prestigious December ... [Last Updated On: May 4th, 2015] [Originally Added On: December 5th, 2012]
- Andropause: Changes in Aging Men - Video [Last Updated On: December 10th, 2012] [Originally Added On: December 10th, 2012]
- Rhein Test Kit - Avante Medical Center - Video [Last Updated On: December 10th, 2012] [Originally Added On: December 10th, 2012]
- AM Northwest Appearance - Andropause - Video [Last Updated On: December 10th, 2012] [Originally Added On: December 10th, 2012]
- Lessons in Menopause for Men - Video [Last Updated On: December 15th, 2012] [Originally Added On: December 15th, 2012]
- AAG Health Publishes Testosterone and HGH Discussion-Blog [Last Updated On: May 4th, 2015] [Originally Added On: December 26th, 2012]
- Understanding Andropause: Erectile Dysfunction - Part Two - Video [Last Updated On: February 26th, 2013] [Originally Added On: February 26th, 2013]
- Airing March 2 and 3: BBC World News 30-Minute Segment on Andropause/Aging with Dr. Jeff Life, Healthy Aging Expert ... [Last Updated On: February 28th, 2013] [Originally Added On: February 28th, 2013]
- Andropause - Great Android App For Men - Vigor - Stamina - Video [Last Updated On: February 28th, 2013] [Originally Added On: February 28th, 2013]
- Eastday-Survey finds 1m locals suffer from andropause [Last Updated On: May 4th, 2015] [Originally Added On: May 22nd, 2013]
- What Is Andropause?Diagnosis,Symptoms,Treatment [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Andropause - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Andropause Glossary - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Andropause Symptoms - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Understanding Andropause: Erectile Dysfunction - Part One - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Understanding Andropause - Video [Last Updated On: July 2nd, 2013] [Originally Added On: July 2nd, 2013]
- Andropause video - Video [Last Updated On: July 8th, 2013] [Originally Added On: July 8th, 2013]
- ANDROPAUSE SOLUTION "OSS-TEST" TOP BEST NATURAL TESTOSTERONE BOOSTER - Video [Last Updated On: August 3rd, 2013] [Originally Added On: August 3rd, 2013]
- Natural relief for andropause [Last Updated On: December 2nd, 2017] [Originally Added On: October 10th, 2013]
- Andropause - Causes, Symptoms, Treatment, Diagnosis - Seniors ... [Last Updated On: January 7th, 2018] [Originally Added On: November 3rd, 2013]
- Andropause - Causes, Symptoms, Treatment, Diagnosis - Men's Health ... [Last Updated On: December 31st, 2017] [Originally Added On: November 3rd, 2013]
- Andropause Specialist [Last Updated On: January 21st, 2018] [Originally Added On: November 10th, 2013]
- Understanding Andropause [Last Updated On: October 24th, 2015] [Originally Added On: November 10th, 2013]
- Andropause - Causes, Symptoms, Treatment, Diagnosis - Men's ... [Last Updated On: January 20th, 2018] [Originally Added On: November 18th, 2013]
- Andropause | Male Menopause | Male Menopause Symptoms | Male ... [Last Updated On: November 25th, 2018] [Originally Added On: November 18th, 2013]
- Symptoms of andropause - Men's health [Last Updated On: November 25th, 2018] [Originally Added On: November 21st, 2013]
- Andropause In Men [Last Updated On: December 9th, 2017] [Originally Added On: November 23rd, 2013]
- Andropause - Male Menopause - Androgen Replacement Therapy [Last Updated On: January 13th, 2018] [Originally Added On: November 25th, 2013]
- Andropause 2013 - Reviewed and Ranked - Independent Reviews on ... [Last Updated On: November 25th, 2013] [Originally Added On: November 25th, 2013]
- Andropause, Facts, Symptoms, Diagnosis, Testosterone Treatment [Last Updated On: January 18th, 2018] [Originally Added On: November 27th, 2013]
- Symptoms of Andropause (Male Menopause): Low Testosterone, Low ... [Last Updated On: December 13th, 2017] [Originally Added On: November 27th, 2013]
- Discovery Health "Andropause: Dealing With Male Menopause" [Last Updated On: December 5th, 2017] [Originally Added On: November 27th, 2013]
- Global Toronto's News at Noon Andropause aka "manopause" - Video [Last Updated On: November 27th, 2013] [Originally Added On: November 27th, 2013]
- San Diego Dermatologist Discusses Male Menopause | Andropause Expert in San Diego - Video [Last Updated On: December 7th, 2017] [Originally Added On: December 9th, 2013]
- The American Academy of Anti-Aging Medicine (A4M) Concludes Largest Event in Anti-Aging, Regenerative and Aesthetic ... [Last Updated On: December 19th, 2013] [Originally Added On: December 19th, 2013]
- Andropause Symptoms - Male Menopause Symptoms [Last Updated On: December 16th, 2017] [Originally Added On: December 27th, 2013]
- What Is Andropause? | eHow - eHow | How to Videos, Articles ... [Last Updated On: January 20th, 2018] [Originally Added On: December 30th, 2013]
- Drug companies are pushing that new-man feeling Low T, high stakes [Last Updated On: January 16th, 2014] [Originally Added On: January 16th, 2014]
- Andropause - Male Menopause - Androgen Replacement Therapy ... [Last Updated On: December 24th, 2017] [Originally Added On: January 16th, 2014]
- Male Menopause Symptoms, Treatments, Causes, and More [Last Updated On: January 15th, 2018] [Originally Added On: January 16th, 2014]
- Can you reverse the aging process? [Last Updated On: January 17th, 2014] [Originally Added On: January 17th, 2014]
- Yourwellness Magazine Explores Male Menopause [Last Updated On: January 19th, 2014] [Originally Added On: January 19th, 2014]
- Antiaging Medicine and Research, India Will Highlight “Hormones and Aging in Medical Practice” During 5th Indomedicon ... [Last Updated On: January 24th, 2014] [Originally Added On: January 24th, 2014]
- Care needed when controlling cholesterol [Last Updated On: October 23rd, 2020] [Originally Added On: January 27th, 2014]
- Symptoms of Andropause (Male Menopause): Low Testosterone ... [Last Updated On: October 11th, 2020] [Originally Added On: January 30th, 2014]
- Antiaging Medicine and Research, India Will Highlight Hormones and Aging in Medical Practice During 5th Indomedicon ... [Last Updated On: October 24th, 2020] [Originally Added On: January 31st, 2014]
- The truth about low testosterone and 'male menopause' [Last Updated On: October 31st, 2020] [Originally Added On: January 31st, 2014]
- Surge or shrink [Last Updated On: November 1st, 2020] [Originally Added On: February 11th, 2014]
- How Does Andropause Affect Men? - Video [Last Updated On: November 10th, 2020] [Originally Added On: February 14th, 2014]
- Andropause | Male Menopause | Male Menopause Symptoms ... [Last Updated On: November 25th, 2018] [Originally Added On: February 15th, 2014]
- NuMale Medical - Low Testosterone -- Andropause - Video [Last Updated On: October 21st, 2020] [Originally Added On: February 21st, 2014]
- Boost Your Sex Drive: Solutions For Impotence, Erectile Dysfunction And Low Libido [Last Updated On: May 4th, 2015] [Originally Added On: February 27th, 2014]
- HowStuffWorks "Andropause: Dealing With Male Menopause" [Last Updated On: October 16th, 2020] [Originally Added On: March 7th, 2014]
- The Truth About Andropause: Male Menopause - Explained by Hormone Expert, Dr. Ken G. Knott, MD - Video [Last Updated On: November 20th, 2020] [Originally Added On: March 7th, 2014]
- What Is Andropause? It's Symptoms In Men & Treatment ... [Last Updated On: November 11th, 2020] [Originally Added On: March 14th, 2014]
- Andropause Low Testosterone A Drug Free Natural Approach UnitedMulticare com - Video [Last Updated On: October 9th, 2020] [Originally Added On: March 15th, 2014]
- Low Testosterone (Andropause). What is it? - Video [Last Updated On: October 30th, 2020] [Originally Added On: March 15th, 2014]
- Male menopause is a reality [Last Updated On: October 13th, 2020] [Originally Added On: March 24th, 2014]
- Anatomy Menopause Andropause - Video [Last Updated On: March 25th, 2014] [Originally Added On: March 25th, 2014]
- Menopause & Andropause - Video [Last Updated On: November 7th, 2020] [Originally Added On: March 30th, 2014]
Word Count: 331