Testim 1% (testosterone gel) delivers physiologic amounts of testosterone, producing circulating testosterone levels that approximate normal levels (e.g., 300 1000 ng/dL) seen in healthy men.
Testosterone and dihydrotestosterone (DHT), endogenous androgens, are responsible for normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement; vocal cord thickening; alterations in body musculature; and fat distribution.
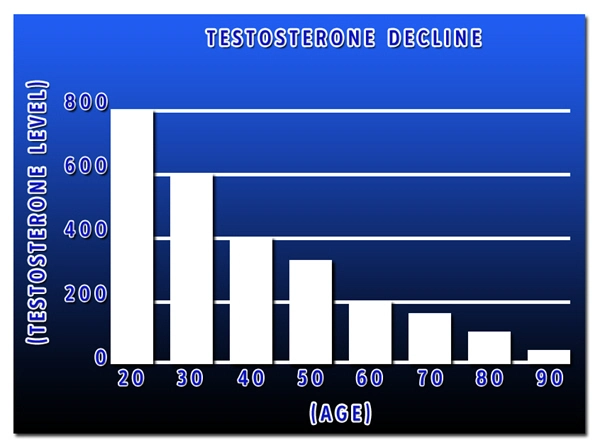
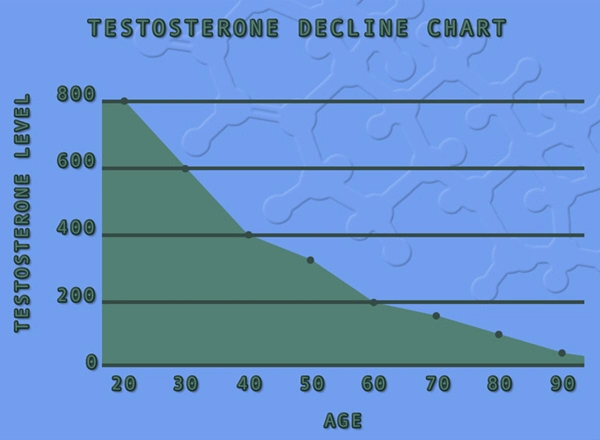
Male hypogonadism results from insufficient secretion of testosterone and is characterized by low serum testosterone concentrations. Symptoms associated with male hypogonadism include decreased sexual desire with or without impotence, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics, and osteoporosis. Hypogonadism is a risk factor for osteoporosis in men.
Drugs in the androgen class also promote retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium.
Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein. Androgens have been reported to stimulate the production of red blood cells by enhancing erythropoietin production.
Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth brought about by fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates but may cause a disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of the growth process.
During exogenous administration of androgens, endogenous testosterone release may be inhibited through feedback inhibition of pituitary luteinizing hormone (LH). At large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH).
There is a lack of substantial evidence that androgens are effective in accelerating fracture healing or in shortening post-surgical convalescence.
The pharmacokinetics of Testim have been evaluated with administration of doses containing 50 mg and 100 mg of testosterone to adult males with morning testosterone levels 300 ng/dL.
Absorption Testim is a topical formulation that dries quickly when applied to the skin surface. The skin serves as a reservoir for the sustained release of testosterone into the systemic circulation. Approximately 10% of the testosterone applied on the skin surface is absorbed into the systemic circulation during a 24-hour period.
Single Dose In single dose studies, when either Testim 50 mg or 100 mg was administered, absorption of testosterone into the blood continued for the entire 24 hour dosing period. Also, mean peak and average serum concentrations within the normal range were achieved within 24 hours.
Multiple Dose With single daily applications of Testim 50 mg and 100 mg, follow-up measurements at 30 and 90 days after starting treatment have confirmed that serum testosterone and DHT concentrations are generally maintained within the normal range.
Figure 1 summarizes the 24-hour pharmacokinetic profile of testosterone for patients maintained on Testim 50 mg or Testim 100 mg for 30 days.
Figure 1 Mean Steady-State Serum Testosterone (SD) (ng/dL) Concentrations on Day 30 in Patients Applying Testim Once Daily
The average daily testosterone concentration produced by Testim 100 mg at Day 30 was 612 ( 286) ng/dL and by Testim 50 mg at Day 30 was 365 ( 187) ng/dL.
Figure 2 summarizes the 24-hour pharmacokinetic profile of DHT for patients maintained on Testim 50 mg or Testim 100 mg for 30 days.
Figure 2 Mean Steady-State Serum Dihydrotestosterone (SD) (pg/mL) Concentrations on Day 30 in Patients Applying Testim Once Daily
The average daily DHT concentration produced by Testim 100 mg at Day 30 was 555 ( 293) pg/mL and by Testim 50 mg at Day 30 was 346 ( 212) pg/mL.
Washing The effect of showering (with mild soap) at 1, 2 and 6 hours post application of Testim 100 mg was evaluated in a clinical trial in 12 men. The study demonstrated that the overall effect of washing was to lessen testosterone levels; however, when washing occurred two or more hours post drug application, serum testosterone levels remained within the normal range.
Circulating testosterone is chiefly bound in the serum to sex hormone-binding globulin (SHBG) and albumin. The albumin-bound fraction of testosterone easily dissociates from albumin and is presumed to be bioactive. The portion of testosterone bound to SHBG is not considered biologically active. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins. The amount of SHBG in the serum and the total testosterone level will determine the distribution of bioactive and nonbioactive androgen.
There is considerable variation in the half-life of testosterone as reported in the literature, ranging from ten to 100 minutes.
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and DHT. Testosterone is metabolized to DHT by steroid 5-reductase located in the skin, liver, and the urogenital tract of the male. DHT binds with greater affinity to SHBG than does testosterone. In many tissues, the activity of testosterone depends on its reduction to DHT, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription and cellular changes related to androgen action. In reproductive tissues, DHT is further metabolized to 3 and 3 androstanediol. Inactivation of testosterone occurs primarily in the liver.
DHT concentrations increased in parallel with testosterone concentrations during Testim treatment. After 90 days of treatment, mean DHT concentrations remained generally within the normal range for Testim-treated subjects.
About 90% of a testosterone dose given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form.
In patients treated with Testim there are no observed differences in the average daily serum testosterone concentration at steady-state based on age or cause of hypogonadism. No formal studies were conducted in a pediatric age population or in patients with renal or hepatic insufficiencies.
Testim was evaluated in a randomized multicenter, multi-dose, active and placebo controlled 90-day study in 406 adult males with morning testosterone levels 300 ng/dL. The study was double-blind for the doses of Testim and placebo, but open label for the non-scrotal testosterone transdermal system. During the first 60 days, patients were evenly randomized to Testim 50 mg, Testim 100 mg, placebo gel, or testosterone transdermal system. At Day 60, patients receiving Testim were maintained at the same dose, or were titrated up or down within their treatment group, based on 24-hour averaged serum testosterone concentration levels obtained on Day 30.
Of 192 hypogonadal men who were appropriately titrated with Testim and who had sufficient data for analysis, 74% achieved an average serum testosterone level within the normal range on treatment Day 90.
Table 1 summarizes the mean testosterone concentrations on Day 30 for patients receiving Testim 50 mg or 100 mg.
At Day 30, patients receiving Testim 100 mg daily showed significant improvement from baseline in multiple sexual function parameters as measured by patient questionnaires when compared to placebo. These parameters included sexual motivation, sexual desire, sexual activity and spontaneous erections. For Testim 100 mg, improvements in sexual motivation, spontaneous erections, and sexual desire were maintained through Day 90. Sexual enjoyment and satisfaction with erection duration were improved compared to baseline but these improvements were not significant compared to the placebo group.
In Testim-treated patients, the number of days in which sexual activity was reported to occur increased by 123% from baseline at Day 30 and was still increased from baseline by 59% at Day 90. The number of days with spontaneous erections increased by 137% at Day 30 and was maintained at 78% at Day 90 for Testim-treated patients compared to baseline.
Table 2 summarizes the changes in body composition at Day 90 for patients receiving Testim 50 mg or 100 mg as measured by standardized whole body DEXA (Dual Energy X-ray Absorptiometry) scanning.
At Day 90, mean increases from baseline in lean body mass and mean decreases from baseline in total fat mass and percent body fat in Testim-treated patients were significant when compared to placebo-treated patients.
Potential for Testosterone Transfer The potential for dermal testosterone transfer following Testim use was evaluated in two clinical trials with males dosed with Testim and their untreated female partners.
In the first trial (AUX-TG-206), 30 couples were evenly randomized to five groups. In the first four groups, 100 mg of Testim was applied to the male abdomen and the couples were then asked to rub abdomen-to-abdomen for 15 minutes at 1 hour, 4 hours, 8 hours or 12 hours after dose application, respectively. In these couples, serum testosterone concentrations in female partners increased from baseline by at least 4 times and potential for transfer was seen at all timepoints.
When 6 males used a shirt to cover the abdomen at 15 minutes post-application and partners again rubbed abdomens for 15 minutes at the 1 hour timepoint, the potential for transfer was markedly reduced.
In the second trial (AUX-TG-209), 24 couples were evenly randomized to four groups. Testim 100 mg was applied to the male arms and shoulders. In one group, 15 minutes of direct skin-to-skin rubbing began at 4 hours after application. In these six women, all of whom showered immediately after the rubbing activity, mean maximum serum testosterone concentrations increased from baseline by approximately 4 times. When males wore a long-sleeved T-shirt and rubbing was started at 1 and at 4 hours after application, the transfer of testosterone from male to female partners was prevented.
See the rest here:
DailyMed - TESTIM - testosterone gel
Contact Us Today For A Free Consultation

- New female 'sex-drive' drug to be tested [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- BioSante will merge with ANI Pharma in stock deal [Last Updated On: December 13th, 2017] [Originally Added On: November 2nd, 2012]
- Female sex-enhancing nasal spray undergoing clinical trials [Last Updated On: October 24th, 2015] [Originally Added On: November 2nd, 2012]
- Project 47 XXY - Living with Klinefelter's Syndrome - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- The Benefits of Testosterone Gel - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- Pros And Cons of Testim - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- BioSante Pharma Completes Enrollment in Pivotal LibiGel Trials - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- Testosterone Replacement and Sperm Production - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- Androgel July 09, 2010, 02:32 PM - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- Insidermedicine In Depth - July 8, 2010 - Testosterone - Video [Last Updated On: November 2nd, 2012] [Originally Added On: November 2nd, 2012]
- A 'female Viagra' nasal spray has started clinical trials [Last Updated On: May 4th, 2015] [Originally Added On: November 9th, 2012]
- GTA IV Heliride - Standing Erection - Video [Last Updated On: December 10th, 2012] [Originally Added On: December 10th, 2012]
- Testosterone gels can affect women, kids [Last Updated On: December 22nd, 2017] [Originally Added On: January 14th, 2013]
- Questions Answers - Detox And Testosterone Gel [Last Updated On: December 29th, 2016] [Originally Added On: August 16th, 2013]
- Testosterone gel: Indications, Side Effects, Warnings - Drugs.com [Last Updated On: December 1st, 2017] [Originally Added On: October 31st, 2013]
- AndroGel® (testosterone gel) 1% - National Institutes of Health [Last Updated On: December 21st, 2017] [Originally Added On: October 31st, 2013]
- What is Testosterone Gel? | Consumer Health PanelConsumer Health Panel [Last Updated On: December 27th, 2017] [Originally Added On: November 15th, 2013]
- What is Testosterone Gel? | Consumer Health PanelConsumer ... [Last Updated On: January 18th, 2018] [Originally Added On: November 18th, 2013]
- AndroGel testosterone gel New FDA Drug Approval | CenterWatch [Last Updated On: January 4th, 2018] [Originally Added On: November 23rd, 2013]
- Testosterone skin gel: Information from Answers.com [Last Updated On: November 28th, 2013] [Originally Added On: November 28th, 2013]
- Testosterone Gels | Do They Work? [Last Updated On: March 28th, 2020] [Originally Added On: December 5th, 2013]
- Testosterone Gel Treatment | eHow - eHow | How to Videos ... [Last Updated On: December 25th, 2017] [Originally Added On: December 6th, 2013]
- Side Effects of AndroGel (Testosterone Gel for Topical Use ... [Last Updated On: December 2nd, 2017] [Originally Added On: December 10th, 2013]
- Female Sexual Desire Drug Rejected by U.S. Regulators [Last Updated On: January 30th, 2018] [Originally Added On: December 12th, 2013]
- Manufacturers of "female Viagra" appeal FDA denial [Last Updated On: December 26th, 2017] [Originally Added On: December 12th, 2013]
- AndroGel (Testosterone Gel for Topical Use) Drug Information ... [Last Updated On: January 23rd, 2018] [Originally Added On: December 24th, 2013]
- Testosterone Gel Vs. Patch | eHow - eHow | How to Videos ... [Last Updated On: December 3rd, 2017] [Originally Added On: December 24th, 2013]
- Testosterone gel: Indications, Side Effects, Warnings ... [Last Updated On: December 18th, 2017] [Originally Added On: January 23rd, 2014]
- Testosterone Treatments Linked to Heart Attacks [Last Updated On: November 28th, 2020] [Originally Added On: January 30th, 2014]
- Testosterone Tx Ups Heart Attack Risk at Any Age [Last Updated On: November 4th, 2020] [Originally Added On: January 31st, 2014]
- Questions & Answers 195 - Video [Last Updated On: November 14th, 2020] [Originally Added On: February 5th, 2014]
- Startling Effects of Testosterone Therapy [Last Updated On: October 29th, 2020] [Originally Added On: February 8th, 2014]
- Group wants heart attack warning on testosterone - Quincy Herald-Whig | Illinois & Missouri News, Sports [Last Updated On: October 16th, 2020] [Originally Added On: February 25th, 2014]
- Could the male hormone testosterone transform a woman's looks, life and libido? [Last Updated On: October 21st, 2020] [Originally Added On: March 6th, 2014]
- Testosterone Monograph for Professionals - Drugs.com [Last Updated On: November 8th, 2020] [Originally Added On: March 7th, 2014]
- Perrigo sues FDA over failure to grant therapeutic equivalence rating for testosterone gel [Last Updated On: October 3rd, 2020] [Originally Added On: March 24th, 2014]
- Low T Litigation - Video [Last Updated On: October 15th, 2020] [Originally Added On: April 2nd, 2014]
- Testosterone Cypionate | Best Testosterone Supplments [Last Updated On: October 15th, 2020] [Originally Added On: April 14th, 2014]
- Is Your Husband At Risk for a Testosterone Heart Attack? [Last Updated On: October 26th, 2020] [Originally Added On: April 16th, 2014]
- How to Apply Testosterone Gel - 31 Day Testosterone Plan - Video [Last Updated On: November 15th, 2020] [Originally Added On: April 22nd, 2014]
- AUXL Gives Bleak Outlook, IG Reverses Loss, ENDP Opens Wallet, BOTA Stops Work [Last Updated On: October 22nd, 2020] [Originally Added On: April 30th, 2014]
- Andractim Testosterone Gel for Testosterone Replacement ... [Last Updated On: October 13th, 2020] [Originally Added On: April 30th, 2014]
- Testosterone Gels [Last Updated On: December 27th, 2017] [Originally Added On: May 1st, 2014]
- Double Blessing For Trimel Pharma... [Last Updated On: May 4th, 2015] [Originally Added On: May 31st, 2014]
- Book Extract: Set ambitious targets to win [Last Updated On: November 7th, 2020] [Originally Added On: June 2nd, 2014]
- Testosterone Nasal Gel Claims To Help Women Reach Orgasm (And Has 'No Side Effects') [Last Updated On: November 30th, 2020] [Originally Added On: June 18th, 2014]
- Wounded veteran is standing tall, looking to the future [Last Updated On: October 6th, 2020] [Originally Added On: July 6th, 2014]
- Wonkblog: Britains economy is finally bigger than it was in 2008. What took so long? [Last Updated On: October 21st, 2020] [Originally Added On: July 26th, 2014]
- As Political Disenchantment Soars, Lines At The Polls Grow Shorter [Last Updated On: November 10th, 2020] [Originally Added On: July 26th, 2014]
- Love Letters: We Don't Have Much To Talk About [Last Updated On: October 28th, 2020] [Originally Added On: July 26th, 2014]
- Why Isn't This Swirling Low in the Atlantic a Tropical Storm? [Last Updated On: November 12th, 2020] [Originally Added On: August 2nd, 2014]
- Don't Make This Big Investing Mistake Once Interest Rates Start Rising [Last Updated On: November 23rd, 2020] [Originally Added On: August 2nd, 2014]
- 'When I look at myself I am not impressed at all' [Last Updated On: October 11th, 2020] [Originally Added On: August 2nd, 2014]
- Androxal superior to Androgel in Phase 3 trial [Last Updated On: October 17th, 2020] [Originally Added On: September 27th, 2014]
- Woman Says Dentists Testosterone Gel Rubbed Off On Her During Sexual Attack, Causing Her To Grow Unsightly Body Hair [Last Updated On: November 13th, 2020] [Originally Added On: September 30th, 2014]
- testosterone gel (Androgel): Drug Facts, Side Effects and ... [Last Updated On: November 16th, 2020] [Originally Added On: October 3rd, 2014]
- Buy Cernos Gel, Buy Testosterone Gel from Certified Online ... [Last Updated On: October 28th, 2014] [Originally Added On: October 28th, 2014]
- AbbVie Sales Blow Past Expectations [Last Updated On: November 30th, 2020] [Originally Added On: October 31st, 2014]
- What Are the Benefits of Testosterone Gel? | eHow [Last Updated On: November 3rd, 2020] [Originally Added On: November 2nd, 2014]
- Secaucus police officer charged with selling steroid gel on eBay: officials [Last Updated On: October 31st, 2020] [Originally Added On: November 18th, 2014]
- Endo Int'l Buys Rights To Natesto Testosterone Nasal Gel From Trimel BioPharma [Last Updated On: October 1st, 2020] [Originally Added On: November 25th, 2014]
- DailyMed - ANDROGEL- testosterone gel [Last Updated On: January 3rd, 2018] [Originally Added On: November 25th, 2014]
- Doctor's Testosterone Gel - Buy Doctors Testosterone Gel ... [Last Updated On: November 24th, 2020] [Originally Added On: December 28th, 2014]
- Testosterone Gel A Convenient, Natural Solution [Last Updated On: October 24th, 2015] [Originally Added On: December 30th, 2014]
- Gilead rival AbbVie's new hepatitis C treatment wins EU approval [Last Updated On: November 28th, 2020] [Originally Added On: January 20th, 2015]
- Men, women: Why cholesterol matters [Last Updated On: October 28th, 2020] [Originally Added On: January 30th, 2015]
- Vital Health, Inc. Provides Tips On How To Combat Cystic Acne Through Dietary Changes and Natural Remedies [Last Updated On: May 4th, 2015] [Originally Added On: February 20th, 2015]
- Higher heart disease risk for men due to high testosterone, low estrogen [Last Updated On: May 4th, 2015] [Originally Added On: March 9th, 2015]
- Men's heart disease risk linked to high testosterone and low estrogen [Last Updated On: November 27th, 2020] [Originally Added On: March 9th, 2015]
- High testosterone ups heart disease risk in men [Last Updated On: May 4th, 2015] [Originally Added On: March 10th, 2015]
- Testosterone Gel - How RS Transaderm makes your test ... [Last Updated On: November 15th, 2020] [Originally Added On: March 12th, 2015]
- Low T Treatment | FORTESTA (testosterone) Gel CIII [Last Updated On: October 19th, 2020] [Originally Added On: March 17th, 2015]
- Why Repros Therapeutics, Inc. Shares Are Soaring [Last Updated On: October 5th, 2020] [Originally Added On: April 2nd, 2015]
- Testosterone Gel Treatment of Gynecomastia | eHow [Last Updated On: January 1st, 2018] [Originally Added On: April 6th, 2015]
- DailyMed - TESTOSTERONE- testosterone gel, metered [Last Updated On: October 22nd, 2020] [Originally Added On: July 3rd, 2015]
- Testim gel: Indications, Side Effects, Warnings - Drugs.com [Last Updated On: October 19th, 2020] [Originally Added On: July 3rd, 2015]
- Testim (Testosterone Gel) Drug Information: Description ... [Last Updated On: October 25th, 2020] [Originally Added On: July 3rd, 2015]
- AndroGel® (Testosterone Gel) 1.62% [Last Updated On: December 11th, 2017] [Originally Added On: August 2nd, 2015]
- AndroGel (testosterone gel) [Last Updated On: December 28th, 2017] [Originally Added On: August 12th, 2015]
- List Of Low-Carb Doctors [Last Updated On: October 10th, 2020] [Originally Added On: September 25th, 2015]
Word Count: 1524