Androgen, also called androgenic hormone or testoid, is the generic term for any natural or synthetic compound, usually a steroid hormone, that stimulates or controls the development and maintenance of male characteristics in vertebrates by binding to androgen receptors.[1] This includes the activity of the accessory male sex organs and development of male secondary sex characteristics. Androgens were first discovered in 1936. Androgens are also the original anabolic steroids and the precursor of all estrogens. The primary and most well-known androgen is testosterone. Dihydrotestosterone (DHT) and androstenedione are less known generally, but are of equal importance in male development. DHT in the embryo life causes differentiation of penis, scrotum and prostate. Later in life DHT contributes to male balding, prostate growth and sebaceous gland activity.
A subset of androgens, adrenal androgens, includes any of the 19-carbon steroids synthesized by the adrenal cortex, the innermost layer of the adrenal cortex (zonula reticularis), that function as weak steroids or steroid precursors, including dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione.
Besides testosterone, other androgens include:
During mammalian development, the gonads are at first capable of becoming either ovaries or testes.[3] In humans, starting at about week 4, the gonadal rudiments are present within the intermediate mesoderm adjacent to the developing kidneys. At about week 6, epithelial sex cords develop within the forming testes and incorporate the germ cells as they migrate into the gonads. In males, certain Y chromosome genes, particularly SRY, control development of the male phenotype, including conversion of the early bipotential gonad into testes. In males, the sex cords fully invade the developing gonads.
The mesoderm-derived epithelial cells of the sex cords in developing testes become the Sertoli cells, which will function to support sperm cell formation. A minor population of nonepithelial cells appear between the tubules by week 8 of human fetal development. These are Leydig cells. Soon after they differentiate, Leydig cells begin to produce androgens.
The androgens function as paracrine hormones required by the Sertoli cells to support sperm production. They are also required for masculinization of the developing male fetus (including penis and scrotum formation). Under the influence of androgens, remnants of the mesonephron, the Wolffian ducts, develop into the epididymis, vas deferens and seminal vesicles. This action of androgens is supported by a hormone from Sertoli cells, Mllerian inhibitory hormone (MIH), which prevents the embryonic Mllerian ducts from developing into fallopian tubes and other female reproductive tract tissues in male embryos. MIH and androgens cooperate to allow for the normal movement of testes into the scrotum.
Before the production of the pituitary hormone luteinizing hormone (LH) by the embryo starting at about weeks 1112, human chorionic gonadotrophin (hCG) promotes the differentiation of Leydig cells and their production of androgens at week 8. Androgen action in target tissues often involves conversion of testosterone to 5-dihydrotestosterone (DHT).
During puberty, androgen, LH and follicle stimulating hormone (FSH) production increase and the sex cords hollow out, forming the seminiferous tubules, and the germ cells start to differentiate into sperm. Throughout adulthood, androgens and FSH cooperatively act on Sertoli cells in the testes to support sperm production.[4] Exogenous androgen supplements can be used as a male contraceptive. Elevated androgen levels caused by use of androgen supplements can inhibit production of LH and block production of endogenous androgens by Leydig cells. Without the locally high levels of androgens in testes due to androgen production by Leydig cells, the seminiferous tubules can degenerate, resulting in infertility. For this reason, many transdermal androgen patches are applied to the scrotum.
Males typically have less body fat than females. Recent results indicate androgens inhibit the ability of some fat cells to store lipids by blocking a signal transduction pathway that normally supports adipocyte function.[5] Also, androgens, but not estrogens, increase beta adrenergic receptors while decreasing alpha adrenergic receptors- which results in increased levels of epinephrine/ norepinephrine due to lack of alpha-2 receptor negative feedback and decreased fat accumulation due to epinephrine/ norepinephrine then acting on lipolysis-inducing beta receptors.
Males typically have more skeletal muscle mass than females. Androgens promote the enlargement of skeletal muscle cells and probably act in a coordinated manner to function by acting on several cell types in skeletal muscle tissue.[6] One cell type conveys hormone signals to generating muscle, the myoblast. Higher androgen levels lead to increased expression of androgen receptor. Fusion of myoblasts generates myotubes, in a process linked to androgen receptor levels.[7]
See the rest here:
Androgen - Wikipedia, the free encyclopedia
Contact Us Today For A Free Consultation

- Adverse Effects of Testosterone Therapy in Adult Men: A Systematic Review and Meta-Analysis [Last Updated On: July 2nd, 2024] [Originally Added On: June 4th, 2010]
- Low Testosterone Levels, Foods That Increase Testosterone Levels wwwSelf-Improvement-Bible.com [Last Updated On: November 12th, 2023] [Originally Added On: May 30th, 2011]
- Low Testosterone in Men: The Next Big Thing in Medicine! - Abraham Morgentaler, MD [Last Updated On: May 7th, 2023] [Originally Added On: June 3rd, 2011]
- How To Determine Testosterone Levels By Looking At Your Ring Finger [Last Updated On: December 7th, 2017] [Originally Added On: June 30th, 2011]
- Prolab Horny Goat Weed Testosterone Booster Supplement Review [Last Updated On: November 23rd, 2023] [Originally Added On: July 19th, 2011]
- The Healthy Skeptic: Products make testosterone claims [Last Updated On: August 13th, 2024] [Originally Added On: September 11th, 2011]
- How To Naturally Increase Testosterone [Last Updated On: November 21st, 2023] [Originally Added On: September 28th, 2011]
- Testosterone Production - Video [Last Updated On: November 25th, 2024] [Originally Added On: November 20th, 2011]
- Testosterone makes us less cooperative and more egocentric, study finds [Last Updated On: January 23rd, 2018] [Originally Added On: February 1st, 2012]
- Testosterone makes us less cooperative and more egocentric [Last Updated On: January 24th, 2018] [Originally Added On: February 1st, 2012]
- Too much testosterone makes for bad decisions, tests show [Last Updated On: April 30th, 2025] [Originally Added On: February 1st, 2012]
- Today in Research: Testosterone's Negative Effects; Diet Soda Death [Last Updated On: January 2nd, 2018] [Originally Added On: February 2nd, 2012]
- Testosterone drives ego, trips cooperation [Last Updated On: December 2nd, 2017] [Originally Added On: February 4th, 2012]
- FDA approves BioSante/Teva's testosterone gel [Last Updated On: April 28th, 2025] [Originally Added On: February 15th, 2012]
- 'Manly' Fingers Make For Strong Jawline in Young Boys [Last Updated On: December 1st, 2017] [Originally Added On: February 15th, 2012]
- Teva, BioSante Win U.S. Approval for Testosterone Therapy [Last Updated On: December 10th, 2017] [Originally Added On: February 15th, 2012]
- BioSante Gains on Approval of Testosterone Gel: Chicago Mover [Last Updated On: January 8th, 2018] [Originally Added On: February 16th, 2012]
- BioSante soars following drug approval from FDA [Last Updated On: December 26th, 2017] [Originally Added On: February 16th, 2012]
- Antibodies, Not Hard Bodies: The Real Reason Women Drool Over Brad Pitt [Last Updated On: December 24th, 2017] [Originally Added On: February 21st, 2012]
- Almark Publishing Releases Book From Mark Rosenberg, M.D. Revealing Natural Discoveries Associated With Low ... [Last Updated On: May 3rd, 2025] [Originally Added On: February 28th, 2012]
- Testosterone Replacement Clinic Comes to Kansas City with Potential to Help Thousands of Men [Last Updated On: May 2nd, 2025] [Originally Added On: March 1st, 2012]
- Study examines the relative roles of testosterone and its metabolite, dihydrotestosterone in men [Last Updated On: December 2nd, 2017] [Originally Added On: March 7th, 2012]
- The Role of 5{alpha}-Reductase Inhibition in Men Receiving Testosterone Replacement Therapy [Editorial] [Last Updated On: December 21st, 2017] [Originally Added On: March 7th, 2012]
- Effect of Testosterone Supplementation With and Without a Dual 5{alpha}-Reductase Inhibitor on Fat-Free Mass in Men ... [Last Updated On: January 3rd, 2018] [Originally Added On: March 7th, 2012]
- Why We Like Men Who Can Keep Their Cool [Last Updated On: December 30th, 2017] [Originally Added On: March 7th, 2012]
- Testosterone And Heart Health [Last Updated On: May 1st, 2025] [Originally Added On: March 10th, 2012]
- Your Life on Testosterone: Overly Sure of Yourself, Unwilling to Listen [Last Updated On: November 25th, 2018] [Originally Added On: March 15th, 2012]
- Mayo Clinic-TGen study role testosterone may play in triple negative breast cancer [Last Updated On: December 8th, 2017] [Originally Added On: March 23rd, 2012]
- A dose of testosterone might not cure what ails you [Last Updated On: January 23rd, 2018] [Originally Added On: March 25th, 2012]
- Green tea could aid athletes hide testosterone doping [Last Updated On: December 16th, 2017] [Originally Added On: March 25th, 2012]
- TGen Study Role Testosterone May Play in Triple Negative Breast Cancer [Last Updated On: December 6th, 2017] [Originally Added On: March 26th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe [Last Updated On: January 23rd, 2018] [Originally Added On: March 28th, 2012]
- Competition-linked bursts of testosterone are fundamental aspect of human biology, study of Amazonian tribe suggests [Last Updated On: December 25th, 2017] [Originally Added On: March 28th, 2012]
- Playing football boosts testosterone levels by 30 percent! [Last Updated On: February 4th, 2024] [Originally Added On: March 28th, 2012]
- Testosterone low, but responsive to competition, in Amazonian tribe -- with slideshow [Last Updated On: December 9th, 2017] [Originally Added On: March 28th, 2012]
- The benefits of testosterone pellet therapy [Last Updated On: January 24th, 2018] [Originally Added On: March 29th, 2012]
- Low testosterone levels cause health woes [Last Updated On: November 25th, 2018] [Originally Added On: March 30th, 2012]
- Heart Failure Patients Getting Relief from Testosterone Supplements [Last Updated On: May 5th, 2025] [Originally Added On: April 21st, 2012]
- Study Finds Fatherhood Suppresses Testosterone [Last Updated On: May 4th, 2025] [Originally Added On: May 3rd, 2012]
- Low testosterone levels could raise diabetes risk for men [Last Updated On: January 26th, 2018] [Originally Added On: May 5th, 2012]
- Why low testosterone may increase your risk of diabetes [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Diabetes link to low testosterone [Last Updated On: November 25th, 2024] [Originally Added On: May 5th, 2012]
- Testosterone Linked to Weight Loss in Obese Men [Last Updated On: January 2nd, 2018] [Originally Added On: May 11th, 2012]
- Testosterone may help weight loss [Last Updated On: November 25th, 2024] [Originally Added On: May 11th, 2012]
- Testosterone-fuelled infantile males might be a product of Mom's behaviour [Last Updated On: December 25th, 2017] [Originally Added On: May 11th, 2012]
- Testosterone-fueled infantile males might be a product of Mom's behavior [Last Updated On: January 6th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements may help obese men lose weight [Last Updated On: January 5th, 2018] [Originally Added On: May 11th, 2012]
- Testosterone supplements 'can help men lose their middle-aged spread' [Last Updated On: November 25th, 2024] [Originally Added On: May 12th, 2012]
- Some doctors question safety of testosterone replacement therapy [Last Updated On: January 20th, 2018] [Originally Added On: May 15th, 2012]
- Health Canada Approves New Testosterone Topical Solution for Men [Last Updated On: May 15th, 2025] [Originally Added On: May 15th, 2012]
- Environment trumps genes in testosterone levels, study finds [Last Updated On: May 8th, 2025] [Originally Added On: May 15th, 2012]
- Global Testosterone Replacement Therapy (TRT) Industry [Last Updated On: May 7th, 2025] [Originally Added On: May 21st, 2012]
- Testosterone Fuels Boom, Swindler Sows Panic: Top Business Books [Last Updated On: January 13th, 2018] [Originally Added On: June 2nd, 2012]
- Increase in testosterone drug use [Last Updated On: April 12th, 2018] [Originally Added On: June 4th, 2012]
- Testosterone Promotes Agression Automatically [Last Updated On: January 29th, 2018] [Originally Added On: June 9th, 2012]
- Testosterone shown to help sexually frustrated women [Last Updated On: January 27th, 2018] [Originally Added On: June 9th, 2012]
- Research and Markets: Testosterone Replacement Therapy (TRT) - Global Strategic Business Report [Last Updated On: December 23rd, 2017] [Originally Added On: June 12th, 2012]
- Proposed testosterone testing of some female olympians challenged by Stanford scientists [Last Updated On: January 30th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Makes Bosses Into Jerks, Says Paul Zak [Last Updated On: January 8th, 2018] [Originally Added On: June 14th, 2012]
- Testosterone Therapy: A Misguided Approach to Erectile Dysfunction (ED) [Last Updated On: May 10th, 2025] [Originally Added On: June 20th, 2012]
- New drugs, new ways to target androgens in prostate cancer therapy [Last Updated On: January 8th, 2018] [Originally Added On: June 20th, 2012]
- Long-term testosterone treatment for men results in reduced weight and waist size [Last Updated On: January 19th, 2018] [Originally Added On: June 23rd, 2012]
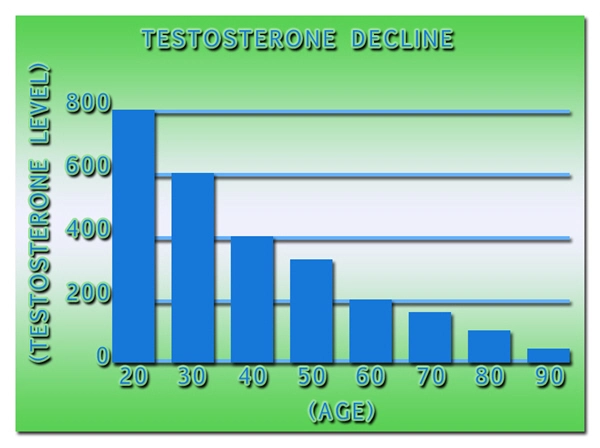
- Declining testosterone levels in men not part of normal aging, study finds [Last Updated On: December 27th, 2017] [Originally Added On: June 23rd, 2012]
- Low testosterone not normal part of aging [Last Updated On: December 22nd, 2017] [Originally Added On: June 25th, 2012]
- Testosterone Does Not Necessarily Wane With Age [Last Updated On: December 6th, 2017] [Originally Added On: June 25th, 2012]
- Overweight men can boost low testosterone levels by losing weight [Last Updated On: December 10th, 2017] [Originally Added On: June 25th, 2012]
- Testosterone-replacement therapy improves symptoms of metabolic syndrome [Last Updated On: January 14th, 2018] [Originally Added On: June 26th, 2012]
- Testosterone therapy takes off pounds [Last Updated On: December 11th, 2017] [Originally Added On: June 26th, 2012]
- Weight loss may boost men's testosterone [Last Updated On: May 9th, 2025] [Originally Added On: June 27th, 2012]
- Low Testosterone? Study finds age may not be to blame [Last Updated On: May 12th, 2025] [Originally Added On: July 1st, 2012]
- Do you have low testosterone? [Last Updated On: December 15th, 2017] [Originally Added On: July 8th, 2012]
- Wall Streeters Buying Testosterone for an Edge [Last Updated On: May 11th, 2025] [Originally Added On: July 12th, 2012]
- Beefy Wall Street Traders rub on testosterone [Last Updated On: February 20th, 2024] [Originally Added On: July 12th, 2012]
- Tale of two runners exposes flawed Olympic thinking [Last Updated On: December 23rd, 2024] [Originally Added On: July 19th, 2012]
- Genetic markers for testosterone and estrogen level regulation identified [Last Updated On: January 6th, 2018] [Originally Added On: July 20th, 2012]
- BUSM researchers identify genetic markers for testosterone, estrogen level regulation [Last Updated On: December 18th, 2017] [Originally Added On: July 20th, 2012]
- DRS. OZ AND ROIZEN: How to reap the benefits of normal testosterone levels [Last Updated On: December 23rd, 2024] [Originally Added On: July 21st, 2012]
- How Testosterone Drives History [Last Updated On: December 24th, 2024] [Originally Added On: July 22nd, 2012]
- Testosterone replacement is "fountain of youth" for men [Last Updated On: January 3rd, 2018] [Originally Added On: July 27th, 2012]
- Pill for low testosterone in men heads for phase II clinical trials [Last Updated On: December 31st, 2017] [Originally Added On: August 2nd, 2012]
Word Count: 737